|
|
Build Your Online Product Catalogs?
| Product Name: |
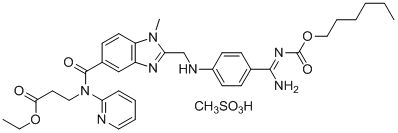
Dabigatran etexilate mesylate
|
| Supply Ability: |
|
| Related proudcts |
pradaxa, |
| Specifications |
99%+ purity, unknown single impurity<0.1% |
| Price Term: |
according to the customers' requests |
| Port of loading: |
according to the customers' requests |
| Minimum Order |
|
| Unit Price: |
|
|
| Dabigatran etexilate mesylate was first approved by EMA on May 18,2008, then approved by the USA FDA on Oct 19,2010, and approved by P*** on Jan 21,2011. It was developed and marketed as Pradaxa by Boehringer Ingelheim in DE and GB. Blood system API; Anti-clotting drugs; Used in patients with nonvalvular atrial fibrillation reduce the risk of stroke and systemic embolism. Darby and group of ester is a new kind of synthesis of direct thrombin inhibitor, is the precursor of dabigatran, of non peptide thrombin inhibitors. Oral gastrointestinal absorption. |
| Company: |
Shenzhen Nexconn Pharmatechs Ltd
|
| Contact: |
Mr. Jason Scorpion |
| Address: |
Rm.1249-1251, Jiaxiye Plaza, No.318 Minzhi Ave., Longhua New Dist |
| Postcode: |
518131 |
| Tel: |
+86-755-89396905 |
| Fax: |
+86-755-22712255 |
| E-mail: |

|
|
|
|