|
|
Build Your Online Product Catalogs?
| Product Name: |
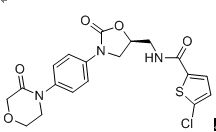
Rivaroxaban
|
| Supply Ability: |
|
| Related proudcts |
|
| Specifications |
99%+ purity, unknown single impurity<0.1% |
| Price Term: |
according to the customers' request |
| Port of loading: |
according to the customers' request |
| Minimum Order |
|
| Unit Price: |
|
|
Rivaroxaban was first approved by Health Canada on September 15, 2008, then approved by European Medicine AgencyŁ¨EMAŁ©on September 30, 2008, and approved by the US Food and Drug AdministrationŁ¨FDAŁ© on July 01,2011. It was developed by Bayer and Janssen, then marketed as Xarelto by Bayer Pharma. In CA and EU and by Janssen in the US.
Rivaroxaban is a selective inhibitor of FXa. It inhibits both free Factor Xa and Factor Xa bound in the prothrombinase complex. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation. Xarelto co-administered with acetylsalicylic acid(ASA) alone or with ASA plus clopidogrel or ticlopidine, is indicated for the prevention of atherothrombotic events in adult patients after an acute coronary syndrome(ACS) with elevated cardiac biomarkers.
|
| Company: |
Shenzhen Nexconn Pharmatechs Ltd
|
| Contact: |
Mr. Jason Scorpion |
| Address: |
Rm.1249-1251, Jiaxiye Plaza, No.318 Minzhi Ave., Longhua New Dist |
| Postcode: |
518131 |
| Tel: |
+86-755-89396905 |
| Fax: |
+86-755-22712255 |
| E-mail: |

|
|
|
|